On September 13, 2023, ASHP held its annual Legislative Day in Washington, DC. Participants from Kansas had the opportunity to advocate for our patients and our profession related to the following key legislative priorities:
- Patient access to pharmacist services
- 340B pricing
- “Site-neutral” payments for infusion services
- Drug shortages
- Pharmacists treating opioid use disorder
- Pharmacy residency funding
Reinforcing our “ask” of legislators is a meaningful method of underscoring the importance of these messages. Following is a quick-reference for specific bills of interest, highlights of each advocacy issue, and more. Contact information for each Congressperson and Representative is also included.
Patient Access
The Equitable Community Access to Pharmacists Services Act (H.R. 1770, S. 2477) allows Medicare patients access to pharmacist-driven test-to-treat services. Without these protections, piecemeal solutions that reimburse pharmacist services create a legal disparity between Medicare recipients and others. Targeted support for test-to-treat gets a foot in the door for more expansive measures, such as The Pharmacy and Medically Underserved Areas Enhancement Act (S. 1491). Kansas pharmacists have been able to become signatories to our statewide test-to-treat protocol since March 2023 (K.S.A. 65-16,131), which encompasses, influenza, streptococcal pharyngitis, and urinary tract infections.
340B Pricing
Protecting access to robust 340B pharmacy services is an essential component of pharmacoequity in the current healthcare model. The PROTECT 340B Act (H.R. 2534) prohibits pharmacy benefits managers (PBMs), drug manufacturers, and other entities from enacting discriminatory policies. In the past, these actions have included under-paying for 340B medications and enacting onerous reporting requirements meant to disincentivize 340B participation. These barriers also threaten the myriad related services provided in 340B sites, such as free immunizations, medication therapy management, and patient assistance programs – particularly detrimental to patients residing in areas dependent on safety net hospitals and who are un- or under-insured. No equivalent Senate bill exists, but the pharmacy workforce advocates can ask for support for parallel bills and request support for Health and Human Services (HHS) enforcement of existing regulations.
Site-Neutral Payments
Draft legislation of the Primary Care and Health Workforce Expansion Act, the PATIENT Act of 2023 (H.R. 3561), and the Healthcare Price Transparency Act (H.R. 4822) include provisions that reimburse “neutrally” for infusion care, irrespective of site of administration, ie physicians’ offices versus hospitals. ASHP rejects this model due to the objectively different standards of care provided in the hospital setting. When medications are infused in the hospital, additional measures are taken for safe preparation, safe administration, care coordination, and safety oversight; these steps (eg clean-room compounding, pharmacist supervision of drug preparation, drug barcoding, on-site pharmacy services) necessarily establish a higher level of care and should be reimbursed accordingly.
Drug Shortages
The pharmacy workforce is acutely aware of the harm and hardship caused by seemingly unrelenting drug shortages. While no specific bill exists that addresses this multi-faceted challenge, ASHP has compiled a comprehensive list of policy solutions that would target key drivers of constrained medication supply. Tackling these root causes would help avoid preventable major impacts on patient care, such as medication rationing, delayed care, and excessive costs.
• Enforce existing shortage prevention requirements – amend section 510(j) of the FDCA to penalize manufacturers that fail to plan for shortages as required by law
• Improve transparency into manufacturer quality – publicize metrics of quality manufacturing maturity (QMM) and un-redacted manufacturing inspection reports to allow purchasers to preferentially select more reliable manufacturers
• Encourage new manufacturers and manufacturing sites – waive generic user fees for generic drugs for manufacturers that commit to market their generic drug if approved (per 506C(g) of FDCA, via ANDA)
• Encourage long-term, guaranteed-volume contracts – provide “add-on” payments when manufacturers commit to acquiring at least 50% of historical purchase volume for products via long-term contracts; applies to selected products as defined by HHS
• Diversify the manufacturing base – encourage supply-chain redundancy by diversifying federal purchase across at least three different manufacturers for critical generic drugs as identified by HHS to be at-risk for shortage
• Finance private sector buffer supplies – finance private-sector buffer inventories at low- or no-cost
Treating Opioid Use Disorder (OUD)
The Fentanyl Safe Testing and Overdose Prevention Act (S. 2569) and Test Strip Access Act of 2023 (H.R. 4106) focus on medications for OUD (MOUD) and harm reduction strategies to help prevent patient morbidity and mortality related to this diagnosis. After elimination of the X-waiver – an example of successful pharmacy workforce advocacy – significant public health barriers continue to persist. Pharmacists’ clinical expertise pertaining to pain management, MOUD, and social determinants of health are invaluable in this effort. The HHS could leverage the Public Readiness and Emergency Preparedness (PREP) Act for the OUD public health emergency, just as it did for the COVID pandemic to allow pharmacists greater latitude to intervene for our patients. Of particular note, fentanyl test strips have been decriminalized in Kansas with broad bipartisan support since May 2023.
Residency Funding
A long-standing ask, ASHP requests that CMS provide transparency in the agency’s requirements for residency programs to receive pass-through funding. Ambiguity about current standards lead to funding clawbacks, creating a moving target for compliance and jeopardizing the sustainability of these essential post-graduate training programs. ASHP asks that CMS suspend cost disallowances until clear guidance is provided.
Kansas Senators (Federal)
Jerry Moran – R
(202) 224-6521, moran.senate.gov
Roger Marshall – R
(202) 224-4774, marshall.senate.gov
Representatives (Federal)
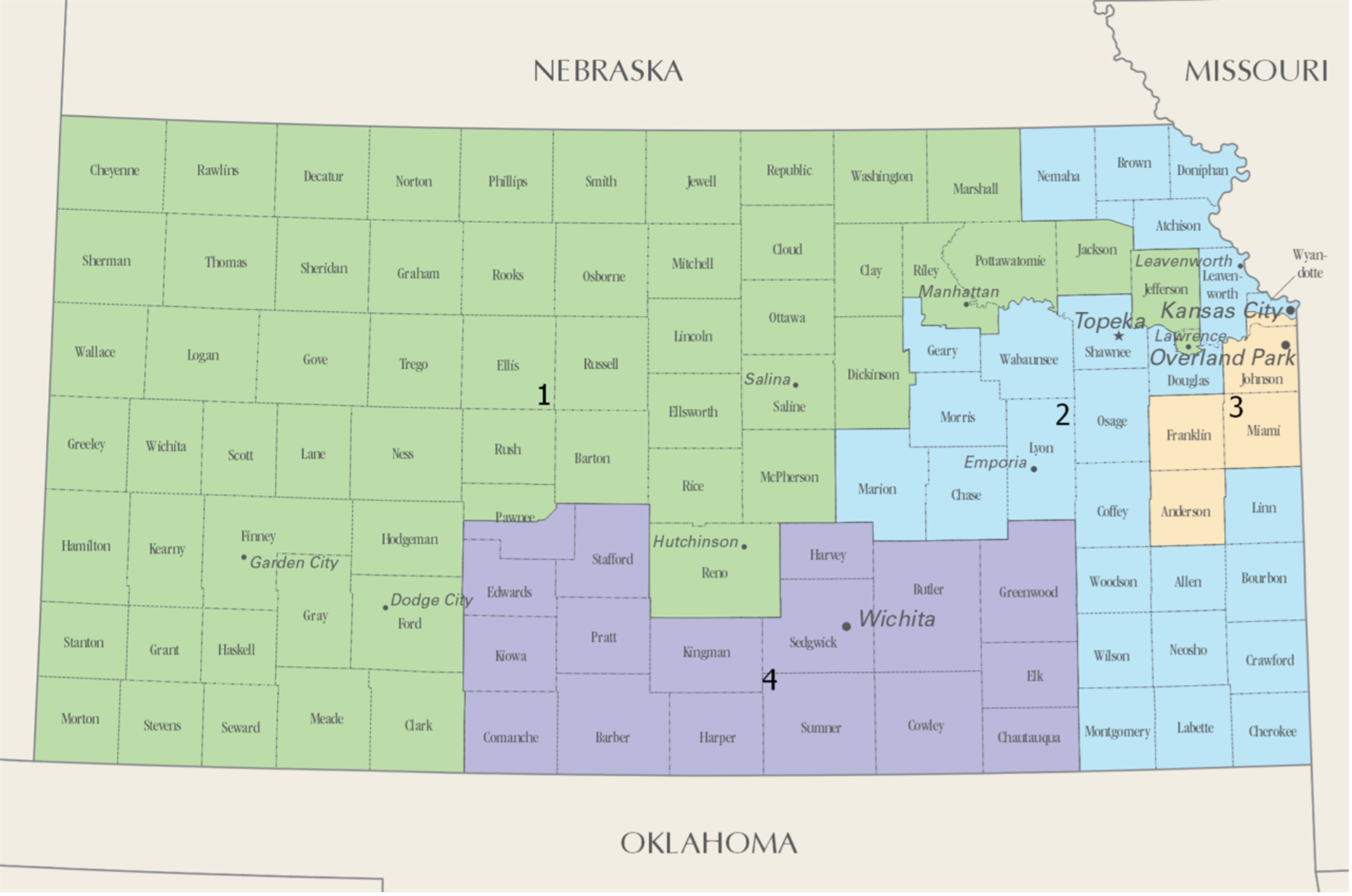
Tracey Mann – R (1st District)
(202) 225-2715, mann.house.gov
Jake LaTurner – R (2nd District)
(202) 225-6601, laturner.house.gov
Sharice Davids – D (3rd District)
(202) 225-2865, davids.house.gov
Ron Estes – R (4th District)
(202) 225-6216, estes.house.gov
Kansas Congressional Districts